Whether the medical equipment of the hospital safe? Who is responsible for the problem ? How to safeguard rights when the accident happen? The newly revised"Medical Devices Regulations"established the most stringent regulatory system covering the whole process.And long before the"Regulations" release,the State Food and Drug Administration has already begun work on rectifying the cheap lead glasses medical device industry.From mid-March,for a period of five months of medical devices"Five remediation"special action,carried out in the country,focusing on regulation of medical devices registered false reporting,illegal production and illegal business,hype,the use of unlicensed products and so on. Sampling,strong inspection can not be guaranteed,it lay hidden for safety Worked in the medical device industry more than ten years Mr.Xu believes that lack sophisticated technology medical device industry,the overall industrial backwardness,is an important cause of domestic medical equipment industry worse than abroad.But in Chinese Medicine Association of Medical Device Materials Branch Secretary Chen Hongyan seems that the threshold is too low,lax supervision,lazy Responsibility chaos as a result of the medical device industry is currently facing the biggest problem.  Under the existing regulations,all medical device companies need to apply to the competent agency for"Medical Device Manufacturing Enterprise License"before you can apply for production licenses in the local business sector.Before the products get out the factory,this should be carried out in the factory in the inspection,sampling and accept the country,after the quality standards in order to enter the hands of the operators,by their products to sales terminals.However,some small businesses ignore internal inspection,sampling escaped the country after he met the acceptance lax operators,it will lead to poor quality products all the way to the green,to the market. Director of Department of Peking University People's Hospital Equipment Shen Chen Yang told reporters,when the device enters into the use of medical institutions,belongs to the state measurement detection range must undergo a compulsory annual measuring and testing to ensure its quality.However,Mr.Xu said that in some grassroots small hospital,because of poor management,can not guarantee strong inspection once a year,causing a problem of extended service of medical devices,planted health risks to users. Medical institutions,strong sense of responsibility for the patient to recover "Although the quality of medical equipment,related to the life and health,but in the rights,whether institutions or the people themselves,are not strong enough sense of Responsibility."For example,you bought a home therapy device,found the problem to find manufacturers,manufacturers may apart from anything else and then replace it give you,you would not bother to chase a responsibility;If you buy a mask,Band-Aid kind of low- value products,if found good,that is most readily throw away,even the manufacturers are not look for.In the medical institutions,large hospitals simply do not survive inferior equipment,and small organizations to buy quality if there are problems,that is,directly to the manufacturer for repair or replacement.Events occur as long as there is no real threat to the life,inferior products will be able to muddle through,the manufacturer will be able to avoid liability. It is understood that after the implementation of the"Medical Devices Regulations"issued in 2000,10 years,with the development and changes in China's medical equipment industry,which is not suitable for development of the industry situation even more prominent.For example,it is responsible for the chase would not provide details on the terms of the lack of bad product traceability system. The newly revised"Supervision and Regulation of Medical Devices"provides medical equipment enterprises,and use of medical equipment purchased should pass inspection documents the supplier qualification and medical equipment,establish inspection record system.The second class,third class medical equipment wholesale business as well as Class III medical devices retail business ventures,sales records system should also be established.Medical equipment unit shall keep the original data acquired Class III medical devices,and to ensure that the information traceability. No matter what the problem is now in the presence of the medical device market,the new draft amendments are introduced to the industry on a"safety valve",the management of medical devices and drug control in the same position.Of course,the law,also need to be strictly enforced to ensure excellent products,anesthesia ventilator industry health. Production will be the focus of regulation violations With the launch of the special action,the medical device industry consolidation horn sounded.The official said that for a false registration application behavior,the second will focus on remediation,Class III medical devices registration untrue behavior for the first time,carried out in accordance with the provisions of the second,three types of medical equipment for the first time to submit an application for registration of producers of information (focus is on clinical trials report) and verification of the authenticity of tissue samples of the production process,the registration link in the report focus on verification. Production is also a violation of content regulation.Disposable infusion equipment,single-use catheters (package) does not meet the criteria for raw materials and did not seek sterilization,condoms without filing unauthorized commissioned production,hemodialysis concentrates not according to the standard factory inspection,etc.,will be the focus of regulation. In the circulation,illegal operation and will be glued to commence remediation hype behavior.For example,experiential approach to unauthorized sales of the second,three types of medical equipment,unlicensed plain lead eyewear colored decorative contact lenses,hearing aids,did not seek storage and transport in vitro diagnostic reagents and other acts.Again,back pain,myopia,diabetes and hypertension sticking classes,physical therapy medical devices for illegal propaganda;without the approval or approval of the content of tampering unauthorized release illegal advertising,exaggerating the effectiveness and scope of the product;use of medical research institutes or specialists,patient name and image to do to prove the efficacy of advertising and other unlawful acts,etc.. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Who-will-bear-the-responsibility-for-security-of-medical-devices.html
Under the existing regulations,all medical device companies need to apply to the competent agency for"Medical Device Manufacturing Enterprise License"before you can apply for production licenses in the local business sector.Before the products get out the factory,this should be carried out in the factory in the inspection,sampling and accept the country,after the quality standards in order to enter the hands of the operators,by their products to sales terminals.However,some small businesses ignore internal inspection,sampling escaped the country after he met the acceptance lax operators,it will lead to poor quality products all the way to the green,to the market. Director of Department of Peking University People's Hospital Equipment Shen Chen Yang told reporters,when the device enters into the use of medical institutions,belongs to the state measurement detection range must undergo a compulsory annual measuring and testing to ensure its quality.However,Mr.Xu said that in some grassroots small hospital,because of poor management,can not guarantee strong inspection once a year,causing a problem of extended service of medical devices,planted health risks to users. Medical institutions,strong sense of responsibility for the patient to recover "Although the quality of medical equipment,related to the life and health,but in the rights,whether institutions or the people themselves,are not strong enough sense of Responsibility."For example,you bought a home therapy device,found the problem to find manufacturers,manufacturers may apart from anything else and then replace it give you,you would not bother to chase a responsibility;If you buy a mask,Band-Aid kind of low- value products,if found good,that is most readily throw away,even the manufacturers are not look for.In the medical institutions,large hospitals simply do not survive inferior equipment,and small organizations to buy quality if there are problems,that is,directly to the manufacturer for repair or replacement.Events occur as long as there is no real threat to the life,inferior products will be able to muddle through,the manufacturer will be able to avoid liability. It is understood that after the implementation of the"Medical Devices Regulations"issued in 2000,10 years,with the development and changes in China's medical equipment industry,which is not suitable for development of the industry situation even more prominent.For example,it is responsible for the chase would not provide details on the terms of the lack of bad product traceability system. The newly revised"Supervision and Regulation of Medical Devices"provides medical equipment enterprises,and use of medical equipment purchased should pass inspection documents the supplier qualification and medical equipment,establish inspection record system.The second class,third class medical equipment wholesale business as well as Class III medical devices retail business ventures,sales records system should also be established.Medical equipment unit shall keep the original data acquired Class III medical devices,and to ensure that the information traceability. No matter what the problem is now in the presence of the medical device market,the new draft amendments are introduced to the industry on a"safety valve",the management of medical devices and drug control in the same position.Of course,the law,also need to be strictly enforced to ensure excellent products,anesthesia ventilator industry health. Production will be the focus of regulation violations With the launch of the special action,the medical device industry consolidation horn sounded.The official said that for a false registration application behavior,the second will focus on remediation,Class III medical devices registration untrue behavior for the first time,carried out in accordance with the provisions of the second,three types of medical equipment for the first time to submit an application for registration of producers of information (focus is on clinical trials report) and verification of the authenticity of tissue samples of the production process,the registration link in the report focus on verification. Production is also a violation of content regulation.Disposable infusion equipment,single-use catheters (package) does not meet the criteria for raw materials and did not seek sterilization,condoms without filing unauthorized commissioned production,hemodialysis concentrates not according to the standard factory inspection,etc.,will be the focus of regulation. In the circulation,illegal operation and will be glued to commence remediation hype behavior.For example,experiential approach to unauthorized sales of the second,three types of medical equipment,unlicensed plain lead eyewear colored decorative contact lenses,hearing aids,did not seek storage and transport in vitro diagnostic reagents and other acts.Again,back pain,myopia,diabetes and hypertension sticking classes,physical therapy medical devices for illegal propaganda;without the approval or approval of the content of tampering unauthorized release illegal advertising,exaggerating the effectiveness and scope of the product;use of medical research institutes or specialists,patient name and image to do to prove the efficacy of advertising and other unlawful acts,etc.. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Who-will-bear-the-responsibility-for-security-of-medical-devices.html
Whether the medical equipment of the hospital safe? Who is responsible for the problem ? How to safeguard rights when the accident happen? The newly revised"Medical Devices Regulations"established the most stringent regulatory system covering the whole process.And long before the"Regulations" release,the State Food and Drug Administration has already begun work on rectifying the cheap lead glasses medical device industry.From mid-March,for a period of five months of medical devices"Five remediation"special action,carried out in the country,focusing on regulation of medical devices registered false reporting,illegal production and illegal business,hype,the use of unlicensed products and so on. Sampling,strong inspection can not be guaranteed,it lay hidden for safety Worked in the medical device industry more than ten years Mr.Xu believes that lack sophisticated technology medical device industry,the overall industrial backwardness,is an important cause of domestic medical equipment industry worse than abroad.But in Chinese Medicine Association of Medical Device Materials Branch Secretary Chen Hongyan seems that the threshold is too low,lax supervision,lazy Responsibility chaos as a result of the medical device industry is currently facing the biggest problem.  Under the existing regulations,all medical device companies need to apply to the competent agency for"Medical Device Manufacturing Enterprise License"before you can apply for production licenses in the local business sector.Before the products get out the factory,this should be carried out in the factory in the inspection,sampling and accept the country,after the quality standards in order to enter the hands of the operators,by their products to sales terminals.However,some small businesses ignore internal inspection,sampling escaped the country after he met the acceptance lax operators,it will lead to poor quality products all the way to the green,to the market. Director of Department of Peking University People's Hospital Equipment Shen Chen Yang told reporters,when the device enters into the use of medical institutions,belongs to the state measurement detection range must undergo a compulsory annual measuring and testing to ensure its quality.However,Mr.Xu said that in some grassroots small hospital,because of poor management,can not guarantee strong inspection once a year,causing a problem of extended service of medical devices,planted health risks to users. Medical institutions,strong sense of responsibility for the patient to recover "Although the quality of medical equipment,related to the life and health,but in the rights,whether institutions or the people themselves,are not strong enough sense of Responsibility."For example,you bought a home therapy device,found the problem to find manufacturers,manufacturers may apart from anything else and then replace it give you,you would not bother to chase a responsibility;If you buy a mask,Band-Aid kind of low- value products,if found good,that is most readily throw away,even the manufacturers are not look for.In the medical institutions,large hospitals simply do not survive inferior equipment,and small organizations to buy quality if there are problems,that is,directly to the manufacturer for repair or replacement.Events occur as long as there is no real threat to the life,inferior products will be able to muddle through,the manufacturer will be able to avoid liability. It is understood that after the implementation of the"Medical Devices Regulations"issued in 2000,10 years,with the development and changes in China's medical equipment industry,which is not suitable for development of the industry situation even more prominent.For example,it is responsible for the chase would not provide details on the terms of the lack of bad product traceability system. The newly revised"Supervision and Regulation of Medical Devices"provides medical equipment enterprises,and use of medical equipment purchased should pass inspection documents the supplier qualification and medical equipment,establish inspection record system.The second class,third class medical equipment wholesale business as well as Class III medical devices retail business ventures,sales records system should also be established.Medical equipment unit shall keep the original data acquired Class III medical devices,and to ensure that the information traceability. No matter what the problem is now in the presence of the medical device market,the new draft amendments are introduced to the industry on a"safety valve",the management of medical devices and drug control in the same position.Of course,the law,also need to be strictly enforced to ensure excellent products,anesthesia ventilator industry health. Production will be the focus of regulation violations With the launch of the special action,the medical device industry consolidation horn sounded.The official said that for a false registration application behavior,the second will focus on remediation,Class III medical devices registration untrue behavior for the first time,carried out in accordance with the provisions of the second,three types of medical equipment for the first time to submit an application for registration of producers of information (focus is on clinical trials report) and verification of the authenticity of tissue samples of the production process,the registration link in the report focus on verification. Production is also a violation of content regulation.Disposable infusion equipment,single-use catheters (package) does not meet the criteria for raw materials and did not seek sterilization,condoms without filing unauthorized commissioned production,hemodialysis concentrates not according to the standard factory inspection,etc.,will be the focus of regulation. In the circulation,illegal operation and will be glued to commence remediation hype behavior.For example,experiential approach to unauthorized sales of the second,three types of medical equipment,unlicensed plain lead eyewear colored decorative contact lenses,hearing aids,did not seek storage and transport in vitro diagnostic reagents and other acts.Again,back pain,myopia,diabetes and hypertension sticking classes,physical therapy medical devices for illegal propaganda;without the approval or approval of the content of tampering unauthorized release illegal advertising,exaggerating the effectiveness and scope of the product;use of medical research institutes or specialists,patient name and image to do to prove the efficacy of advertising and other unlawful acts,etc.. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Who-will-bear-the-responsibility-for-security-of-medical-devices.html
Under the existing regulations,all medical device companies need to apply to the competent agency for"Medical Device Manufacturing Enterprise License"before you can apply for production licenses in the local business sector.Before the products get out the factory,this should be carried out in the factory in the inspection,sampling and accept the country,after the quality standards in order to enter the hands of the operators,by their products to sales terminals.However,some small businesses ignore internal inspection,sampling escaped the country after he met the acceptance lax operators,it will lead to poor quality products all the way to the green,to the market. Director of Department of Peking University People's Hospital Equipment Shen Chen Yang told reporters,when the device enters into the use of medical institutions,belongs to the state measurement detection range must undergo a compulsory annual measuring and testing to ensure its quality.However,Mr.Xu said that in some grassroots small hospital,because of poor management,can not guarantee strong inspection once a year,causing a problem of extended service of medical devices,planted health risks to users. Medical institutions,strong sense of responsibility for the patient to recover "Although the quality of medical equipment,related to the life and health,but in the rights,whether institutions or the people themselves,are not strong enough sense of Responsibility."For example,you bought a home therapy device,found the problem to find manufacturers,manufacturers may apart from anything else and then replace it give you,you would not bother to chase a responsibility;If you buy a mask,Band-Aid kind of low- value products,if found good,that is most readily throw away,even the manufacturers are not look for.In the medical institutions,large hospitals simply do not survive inferior equipment,and small organizations to buy quality if there are problems,that is,directly to the manufacturer for repair or replacement.Events occur as long as there is no real threat to the life,inferior products will be able to muddle through,the manufacturer will be able to avoid liability. It is understood that after the implementation of the"Medical Devices Regulations"issued in 2000,10 years,with the development and changes in China's medical equipment industry,which is not suitable for development of the industry situation even more prominent.For example,it is responsible for the chase would not provide details on the terms of the lack of bad product traceability system. The newly revised"Supervision and Regulation of Medical Devices"provides medical equipment enterprises,and use of medical equipment purchased should pass inspection documents the supplier qualification and medical equipment,establish inspection record system.The second class,third class medical equipment wholesale business as well as Class III medical devices retail business ventures,sales records system should also be established.Medical equipment unit shall keep the original data acquired Class III medical devices,and to ensure that the information traceability. No matter what the problem is now in the presence of the medical device market,the new draft amendments are introduced to the industry on a"safety valve",the management of medical devices and drug control in the same position.Of course,the law,also need to be strictly enforced to ensure excellent products,anesthesia ventilator industry health. Production will be the focus of regulation violations With the launch of the special action,the medical device industry consolidation horn sounded.The official said that for a false registration application behavior,the second will focus on remediation,Class III medical devices registration untrue behavior for the first time,carried out in accordance with the provisions of the second,three types of medical equipment for the first time to submit an application for registration of producers of information (focus is on clinical trials report) and verification of the authenticity of tissue samples of the production process,the registration link in the report focus on verification. Production is also a violation of content regulation.Disposable infusion equipment,single-use catheters (package) does not meet the criteria for raw materials and did not seek sterilization,condoms without filing unauthorized commissioned production,hemodialysis concentrates not according to the standard factory inspection,etc.,will be the focus of regulation. In the circulation,illegal operation and will be glued to commence remediation hype behavior.For example,experiential approach to unauthorized sales of the second,three types of medical equipment,unlicensed plain lead eyewear colored decorative contact lenses,hearing aids,did not seek storage and transport in vitro diagnostic reagents and other acts.Again,back pain,myopia,diabetes and hypertension sticking classes,physical therapy medical devices for illegal propaganda;without the approval or approval of the content of tampering unauthorized release illegal advertising,exaggerating the effectiveness and scope of the product;use of medical research institutes or specialists,patient name and image to do to prove the efficacy of advertising and other unlawful acts,etc.. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Who-will-bear-the-responsibility-for-security-of-medical-devices.html
Sleep apnea is called snoring,which is a disease troubling many people and affects their sleep badly.It is a challenge for medical device manufacturers to cure this disease.In this paper,based on the solution to this problem,we analyzed kinds of transducer solutions uesd in sleep-related breathing equipment and explained the differences among them,Ventilator equipment which provides valuable technical ideas for manufacturers. Sleep apnea syndrome(snoring),which worries millions of patients heavily,refers to breath stop during sleep which can appear repeatedly to a frequency of hundreds of times per night and to a time length of one minutes or more per time.Snoring may leads to memory deterioration and weight imbalance,even hypertension,cardiovascular and cerebrovascular diseases if leave it alone.Besides,the sleep insufficiency and fatigue caused by snoring may turn into the culprit behind inductrial injury accidents and motor vehicle traffic accidents 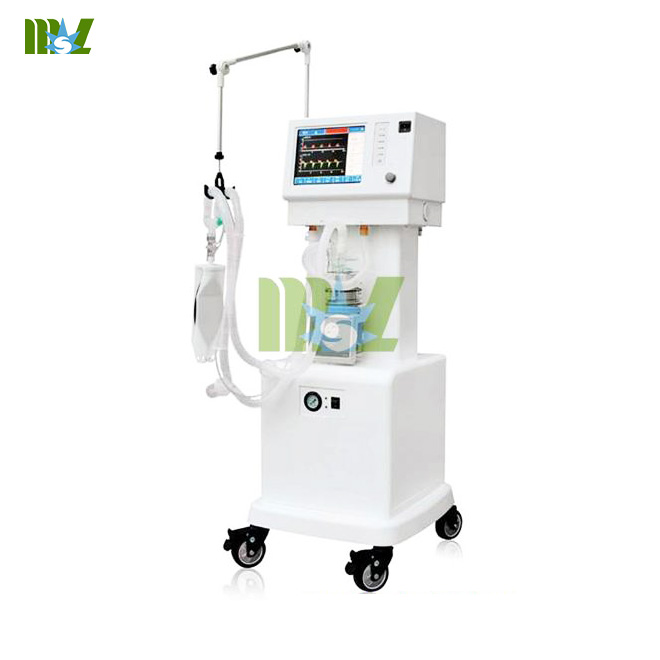 One commonly used method of treatment is using PAP.Patients wear a Special mask when sleeping,which press some air into their Nostrils to prevent Suffocation.There are 3 forms of PAP: *CPAP(continuous positive pressure ventilator)provides definite stressed air continuously.This positive pressure can prevent UAO caused by upper airway collapse,which enables patients to breathe freely in case of apnea *Auto-PAP(automated positive pressure ventilator),can measure the resistance when breathing and adjust the air supply pressure by the magnitude of the resistive force to make sure every breath unimpeded. *Bilevel PAP(bilevel positive pressure ventilator)provides two kinds of pressure:suction of positive pressure and exhalation of positive pressure. The Portable ventilator machine detect the respiratory cycle via a gas mass flow sensor,which comforts patients more.Thus,it's more frequently used than those without this function. The gas mass flow sensor used in the sleep apnea machine must be equipped with high resolution and accurate sensing ability,so that it can sense the weak air change and control the MAF given to patients more accurately.Such sensors should measure the MAF more precisely and at the same time,sense the existence of air flow. Another important consideration is power dissipation.Lower power dissipation makes it possible for the respirator to be powered by battery,which promises more flexibility to the patients Ultimately,the noise produced by motor of the apnea machine should be controlled when patients try to sleep,which is important.It conflicts with the purpose of the Anesthesia Equipments when the noise affects patients' sleep. It's difficult for the motor to work if the sensitivity of pressure drop (pressure drop equals to he resistance of sensor) is too high.Thus a gas mass flow sensor and relatively low pressure drop is needed,or else it will add noise and reduce the motor's working life. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/improve-the-efficiency-accuracy-and-reliability-of-Sleep-apnea-machine.html
One commonly used method of treatment is using PAP.Patients wear a Special mask when sleeping,which press some air into their Nostrils to prevent Suffocation.There are 3 forms of PAP: *CPAP(continuous positive pressure ventilator)provides definite stressed air continuously.This positive pressure can prevent UAO caused by upper airway collapse,which enables patients to breathe freely in case of apnea *Auto-PAP(automated positive pressure ventilator),can measure the resistance when breathing and adjust the air supply pressure by the magnitude of the resistive force to make sure every breath unimpeded. *Bilevel PAP(bilevel positive pressure ventilator)provides two kinds of pressure:suction of positive pressure and exhalation of positive pressure. The Portable ventilator machine detect the respiratory cycle via a gas mass flow sensor,which comforts patients more.Thus,it's more frequently used than those without this function. The gas mass flow sensor used in the sleep apnea machine must be equipped with high resolution and accurate sensing ability,so that it can sense the weak air change and control the MAF given to patients more accurately.Such sensors should measure the MAF more precisely and at the same time,sense the existence of air flow. Another important consideration is power dissipation.Lower power dissipation makes it possible for the respirator to be powered by battery,which promises more flexibility to the patients Ultimately,the noise produced by motor of the apnea machine should be controlled when patients try to sleep,which is important.It conflicts with the purpose of the Anesthesia Equipments when the noise affects patients' sleep. It's difficult for the motor to work if the sensitivity of pressure drop (pressure drop equals to he resistance of sensor) is too high.Thus a gas mass flow sensor and relatively low pressure drop is needed,or else it will add noise and reduce the motor's working life. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/improve-the-efficiency-accuracy-and-reliability-of-Sleep-apnea-machine.html
Sleep apnea is called snoring,which is a disease troubling many people and affects their sleep badly.It is a challenge for medical device manufacturers to cure this disease.In this paper,based on the solution to this problem,we analyzed kinds of transducer solutions uesd in sleep-related breathing equipment and explained the differences among them,Ventilator equipment which provides valuable technical ideas for manufacturers. Sleep apnea syndrome(snoring),which worries millions of patients heavily,refers to breath stop during sleep which can appear repeatedly to a frequency of hundreds of times per night and to a time length of one minutes or more per time.Snoring may leads to memory deterioration and weight imbalance,even hypertension,cardiovascular and cerebrovascular diseases if leave it alone.Besides,the sleep insufficiency and fatigue caused by snoring may turn into the culprit behind inductrial injury accidents and motor vehicle traffic accidents  One commonly used method of treatment is using PAP.Patients wear a Special mask when sleeping,which press some air into their Nostrils to prevent Suffocation.There are 3 forms of PAP: *CPAP(continuous positive pressure ventilator)provides definite stressed air continuously.This positive pressure can prevent UAO caused by upper airway collapse,which enables patients to breathe freely in case of apnea *Auto-PAP(automated positive pressure ventilator),can measure the resistance when breathing and adjust the air supply pressure by the magnitude of the resistive force to make sure every breath unimpeded. *Bilevel PAP(bilevel positive pressure ventilator)provides two kinds of pressure:suction of positive pressure and exhalation of positive pressure. The Portable ventilator machine detect the respiratory cycle via a gas mass flow sensor,which comforts patients more.Thus,it's more frequently used than those without this function. The gas mass flow sensor used in the sleep apnea machine must be equipped with high resolution and accurate sensing ability,so that it can sense the weak air change and control the MAF given to patients more accurately.Such sensors should measure the MAF more precisely and at the same time,sense the existence of air flow. Another important consideration is power dissipation.Lower power dissipation makes it possible for the respirator to be powered by battery,which promises more flexibility to the patients Ultimately,the noise produced by motor of the apnea machine should be controlled when patients try to sleep,which is important.It conflicts with the purpose of the Anesthesia Equipments when the noise affects patients' sleep. It's difficult for the motor to work if the sensitivity of pressure drop (pressure drop equals to he resistance of sensor) is too high.Thus a gas mass flow sensor and relatively low pressure drop is needed,or else it will add noise and reduce the motor's working life. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/improve-the-efficiency-accuracy-and-reliability-of-Sleep-apnea-machine.html
One commonly used method of treatment is using PAP.Patients wear a Special mask when sleeping,which press some air into their Nostrils to prevent Suffocation.There are 3 forms of PAP: *CPAP(continuous positive pressure ventilator)provides definite stressed air continuously.This positive pressure can prevent UAO caused by upper airway collapse,which enables patients to breathe freely in case of apnea *Auto-PAP(automated positive pressure ventilator),can measure the resistance when breathing and adjust the air supply pressure by the magnitude of the resistive force to make sure every breath unimpeded. *Bilevel PAP(bilevel positive pressure ventilator)provides two kinds of pressure:suction of positive pressure and exhalation of positive pressure. The Portable ventilator machine detect the respiratory cycle via a gas mass flow sensor,which comforts patients more.Thus,it's more frequently used than those without this function. The gas mass flow sensor used in the sleep apnea machine must be equipped with high resolution and accurate sensing ability,so that it can sense the weak air change and control the MAF given to patients more accurately.Such sensors should measure the MAF more precisely and at the same time,sense the existence of air flow. Another important consideration is power dissipation.Lower power dissipation makes it possible for the respirator to be powered by battery,which promises more flexibility to the patients Ultimately,the noise produced by motor of the apnea machine should be controlled when patients try to sleep,which is important.It conflicts with the purpose of the Anesthesia Equipments when the noise affects patients' sleep. It's difficult for the motor to work if the sensitivity of pressure drop (pressure drop equals to he resistance of sensor) is too high.Thus a gas mass flow sensor and relatively low pressure drop is needed,or else it will add noise and reduce the motor's working life. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/improve-the-efficiency-accuracy-and-reliability-of-Sleep-apnea-machine.html
Over the past few years,domestic healthcare manufacturing sector getting transformation which objectives require greater ability to benchtop centrifuge.To make sure centrifuge characteristic,healthcare manufacturing need to fulfill requirement to <>.Those devices surface immediate contact with drug should clean,tidy,no death angle,easy to clean or sanitize,corrosion resistance,chemical change or adsorption will not take place with drug. Actually,centrifuge widely used in healthcare sector,especially in hematology separation,virus research,DNA research,medicine refinery.etc.Pharmaceutical Pharmaceutical centrifuge has several features like:flexible,high-automated,operation stable,strong handicraft property,good corrosion resistance,good operating environment,fully safety protection device reliable and attractive appearance etc.Medicine refinery other drugs used in the refining process was generally low-speed centrifuge(High speed centrifuge),rotation rate lower than 4000R,huge throughput,in order to meet drug production GMP specification and requirements,generally use flat stainless steel materials.  Pharmaceutical centrifuge has following advantage from function aspect:1.flexible material,appropriate filter medium not only can separate millimeter fine particle but also can clean article through water pipe.2.Artificial upper unloading type structure is simple,easy installation,low cost,easy to operate,can maintain the shape of the crystal products.3.Machine adopt advanced resilient support structure,can reduce the vibrations caused by uneven load,smooth operate.4.The whole high speed operating structure,concentrated in a closed shell,not only can achieve closed but also avoid contaminate materials.All of Centrifuge corners,the corner welds are ground in the production process had a smooth rounded transition,as much as possible eliminate bump,health corner,effusion,plot materials and other corner.Experts advice:customers choose refrigerated centrifuge suitable materials should according to materials corrosion resistance feature,cleanliness when they choose centrifuge. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Centrifuge-widely-used-in-medical-healthcare-sector.html
Pharmaceutical centrifuge has following advantage from function aspect:1.flexible material,appropriate filter medium not only can separate millimeter fine particle but also can clean article through water pipe.2.Artificial upper unloading type structure is simple,easy installation,low cost,easy to operate,can maintain the shape of the crystal products.3.Machine adopt advanced resilient support structure,can reduce the vibrations caused by uneven load,smooth operate.4.The whole high speed operating structure,concentrated in a closed shell,not only can achieve closed but also avoid contaminate materials.All of Centrifuge corners,the corner welds are ground in the production process had a smooth rounded transition,as much as possible eliminate bump,health corner,effusion,plot materials and other corner.Experts advice:customers choose refrigerated centrifuge suitable materials should according to materials corrosion resistance feature,cleanliness when they choose centrifuge. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Centrifuge-widely-used-in-medical-healthcare-sector.html
Over the past few years,domestic healthcare manufacturing sector getting transformation which objectives require greater ability to benchtop centrifuge.To make sure centrifuge characteristic,healthcare manufacturing need to fulfill requirement to <>.Those devices surface immediate contact with drug should clean,tidy,no death angle,easy to clean or sanitize,corrosion resistance,chemical change or adsorption will not take place with drug. Actually,centrifuge widely used in healthcare sector,especially in hematology separation,virus research,DNA research,medicine refinery.etc.Pharmaceutical Pharmaceutical centrifuge has several features like:flexible,high-automated,operation stable,strong handicraft property,good corrosion resistance,good operating environment,fully safety protection device reliable and attractive appearance etc.Medicine refinery other drugs used in the refining process was generally low-speed centrifuge(High speed centrifuge),rotation rate lower than 4000R,huge throughput,in order to meet drug production GMP specification and requirements,generally use flat stainless steel materials.  Pharmaceutical centrifuge has following advantage from function aspect:1.flexible material,appropriate filter medium not only can separate millimeter fine particle but also can clean article through water pipe.2.Artificial upper unloading type structure is simple,easy installation,low cost,easy to operate,can maintain the shape of the crystal products.3.Machine adopt advanced resilient support structure,can reduce the vibrations caused by uneven load,smooth operate.4.The whole high speed operating structure,concentrated in a closed shell,not only can achieve closed but also avoid contaminate materials.All of Centrifuge corners,the corner welds are ground in the production process had a smooth rounded transition,as much as possible eliminate bump,health corner,effusion,plot materials and other corner.Experts advice:customers choose refrigerated centrifuge suitable materials should according to materials corrosion resistance feature,cleanliness when they choose centrifuge. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Centrifuge-widely-used-in-medical-healthcare-sector.html
Pharmaceutical centrifuge has following advantage from function aspect:1.flexible material,appropriate filter medium not only can separate millimeter fine particle but also can clean article through water pipe.2.Artificial upper unloading type structure is simple,easy installation,low cost,easy to operate,can maintain the shape of the crystal products.3.Machine adopt advanced resilient support structure,can reduce the vibrations caused by uneven load,smooth operate.4.The whole high speed operating structure,concentrated in a closed shell,not only can achieve closed but also avoid contaminate materials.All of Centrifuge corners,the corner welds are ground in the production process had a smooth rounded transition,as much as possible eliminate bump,health corner,effusion,plot materials and other corner.Experts advice:customers choose refrigerated centrifuge suitable materials should according to materials corrosion resistance feature,cleanliness when they choose centrifuge. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Centrifuge-widely-used-in-medical-healthcare-sector.html
As we all know,with the development of the medical technology,the oral diseases,like anisodont,agomphiasis,yellow and black teeth,which are often seen in our common life,is not going to be a big problem.Especially the emergence of dental equipment china dental implant technology,which brings the dental implant tide and make more people regain the oral health and confident smile. First choice for the edentulous person-----dental implant  The common repair methods for the missing teeth including: removable denture repair,fixed denture repair (ceramic teeth),implant denture(dental implant) When the teeth missed,most of people will choose the removable denture,while the removable denture need to be cleaned everyday dental x ray unit,and the chewing ability is poor,the lifespan is short,and also the removable denture will bring some oral disease.The ceramic teeth needs the point of strength from the neighboring teeth,which will damage the neighboring teeth.It will be needed build three dental crown for one missing teeth and also the lifespan is short. Implant tooth is a kind of tooth repair technology rising in the recent years.Planting the Artificial tooth root into the alveolar bone,which will be fused together by this way.The shape is realistic,artistic,comfortable,and the chewing ability is good as the real one.Morever,it won't damage the neighboring teeth and widely accepted by lots of people.So,the implant tooth is also called as the third pair teeth of human.
The common repair methods for the missing teeth including: removable denture repair,fixed denture repair (ceramic teeth),implant denture(dental implant) When the teeth missed,most of people will choose the removable denture,while the removable denture need to be cleaned everyday dental x ray unit,and the chewing ability is poor,the lifespan is short,and also the removable denture will bring some oral disease.The ceramic teeth needs the point of strength from the neighboring teeth,which will damage the neighboring teeth.It will be needed build three dental crown for one missing teeth and also the lifespan is short. Implant tooth is a kind of tooth repair technology rising in the recent years.Planting the Artificial tooth root into the alveolar bone,which will be fused together by this way.The shape is realistic,artistic,comfortable,and the chewing ability is good as the real one.Morever,it won't damage the neighboring teeth and widely accepted by lots of people.So,the implant tooth is also called as the third pair teeth of human.  ) Prussia immediate dental implant make the mutilated teeth grow back. Prussia immediate dental implant is one of the most advanced planting technology in the world,with the characters of precision,stoutness,strong durability,good biocompatibility and short cultivation period.Now it has been brought into China,Prussia immediate dental implant break the traditional long cultivation period of 30 days,only 7-15 days,the cultivation can be finished,for the signal tooth,only 1 hour. Prussia immediate dental implant is applied for signal tooth,several teeth and full teeth,without damaging the neighboring teeth,recover the normal chewing function farthest,the bite force is almost 98.8% of the real tooth.The biological intermiscibility is excellent and the aesthetic effect is great.Nature,comfort,close to the real tooth maximum,no any foreign body sensation,no any effect to the taster.Applying the 3D tooth carving dental unit technology,single crown repair can be finished in one hour,which is high speed and high efficiency.Safe and durable,the first prussian dental implants in the world has been used for almost 40 years. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/which-is-the-best-way-to-cure-the-missing-teeth.html
) Prussia immediate dental implant make the mutilated teeth grow back. Prussia immediate dental implant is one of the most advanced planting technology in the world,with the characters of precision,stoutness,strong durability,good biocompatibility and short cultivation period.Now it has been brought into China,Prussia immediate dental implant break the traditional long cultivation period of 30 days,only 7-15 days,the cultivation can be finished,for the signal tooth,only 1 hour. Prussia immediate dental implant is applied for signal tooth,several teeth and full teeth,without damaging the neighboring teeth,recover the normal chewing function farthest,the bite force is almost 98.8% of the real tooth.The biological intermiscibility is excellent and the aesthetic effect is great.Nature,comfort,close to the real tooth maximum,no any foreign body sensation,no any effect to the taster.Applying the 3D tooth carving dental unit technology,single crown repair can be finished in one hour,which is high speed and high efficiency.Safe and durable,the first prussian dental implants in the world has been used for almost 40 years. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/which-is-the-best-way-to-cure-the-missing-teeth.html
As we all know,with the development of the medical technology,the oral diseases,like anisodont,agomphiasis,yellow and black teeth,which are often seen in our common life,is not going to be a big problem.Especially the emergence of dental equipment china dental implant technology,which brings the dental implant tide and make more people regain the oral health and confident smile. First choice for the edentulous person-----dental implant  The common repair methods for the missing teeth including: removable denture repair,fixed denture repair (ceramic teeth),implant denture(dental implant) When the teeth missed,most of people will choose the removable denture,while the removable denture need to be cleaned everyday dental x ray unit,and the chewing ability is poor,the lifespan is short,and also the removable denture will bring some oral disease.The ceramic teeth needs the point of strength from the neighboring teeth,which will damage the neighboring teeth.It will be needed build three dental crown for one missing teeth and also the lifespan is short. Implant tooth is a kind of tooth repair technology rising in the recent years.Planting the Artificial tooth root into the alveolar bone,which will be fused together by this way.The shape is realistic,artistic,comfortable,and the chewing ability is good as the real one.Morever,it won't damage the neighboring teeth and widely accepted by lots of people.So,the implant tooth is also called as the third pair teeth of human.
The common repair methods for the missing teeth including: removable denture repair,fixed denture repair (ceramic teeth),implant denture(dental implant) When the teeth missed,most of people will choose the removable denture,while the removable denture need to be cleaned everyday dental x ray unit,and the chewing ability is poor,the lifespan is short,and also the removable denture will bring some oral disease.The ceramic teeth needs the point of strength from the neighboring teeth,which will damage the neighboring teeth.It will be needed build three dental crown for one missing teeth and also the lifespan is short. Implant tooth is a kind of tooth repair technology rising in the recent years.Planting the Artificial tooth root into the alveolar bone,which will be fused together by this way.The shape is realistic,artistic,comfortable,and the chewing ability is good as the real one.Morever,it won't damage the neighboring teeth and widely accepted by lots of people.So,the implant tooth is also called as the third pair teeth of human.  ) Prussia immediate dental implant make the mutilated teeth grow back. Prussia immediate dental implant is one of the most advanced planting technology in the world,with the characters of precision,stoutness,strong durability,good biocompatibility and short cultivation period.Now it has been brought into China,Prussia immediate dental implant break the traditional long cultivation period of 30 days,only 7-15 days,the cultivation can be finished,for the signal tooth,only 1 hour. Prussia immediate dental implant is applied for signal tooth,several teeth and full teeth,without damaging the neighboring teeth,recover the normal chewing function farthest,the bite force is almost 98.8% of the real tooth.The biological intermiscibility is excellent and the aesthetic effect is great.Nature,comfort,close to the real tooth maximum,no any foreign body sensation,no any effect to the taster.Applying the 3D tooth carving dental unit technology,single crown repair can be finished in one hour,which is high speed and high efficiency.Safe and durable,the first prussian dental implants in the world has been used for almost 40 years. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/which-is-the-best-way-to-cure-the-missing-teeth.html
) Prussia immediate dental implant make the mutilated teeth grow back. Prussia immediate dental implant is one of the most advanced planting technology in the world,with the characters of precision,stoutness,strong durability,good biocompatibility and short cultivation period.Now it has been brought into China,Prussia immediate dental implant break the traditional long cultivation period of 30 days,only 7-15 days,the cultivation can be finished,for the signal tooth,only 1 hour. Prussia immediate dental implant is applied for signal tooth,several teeth and full teeth,without damaging the neighboring teeth,recover the normal chewing function farthest,the bite force is almost 98.8% of the real tooth.The biological intermiscibility is excellent and the aesthetic effect is great.Nature,comfort,close to the real tooth maximum,no any foreign body sensation,no any effect to the taster.Applying the 3D tooth carving dental unit technology,single crown repair can be finished in one hour,which is high speed and high efficiency.Safe and durable,the first prussian dental implants in the world has been used for almost 40 years. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/which-is-the-best-way-to-cure-the-missing-teeth.html
The kind of catheter MSL introduced on Aug.6th.The innovative feature is that doctors can acquire clearer ultrasonic images(bovine ultrasound machines) when inspecting metrorrhagia patients by injecting saline solution into uterine cavity.During the process,doctors can also sample to do endometrium biopsy as the actual inspection condition may require. Metrorrhagia is one of the most common diseases that lead females to paediatrics and obstetrics.In America,there are up to 10,000,000 patients turn to doctors for metrorrhagia per year.It can be one symptom of some serious illness,such as endometrial carcinoma,leukemia.And even a common metrorrhagia patient can suffer anemia if not timely treated,not to mention that at the same time,daily life of the patient can be affected badly.  It's claimed that the uniquely shaped sealing component of GoldsteinSonoBiopsy can prevent the overflow of saline solution that flows into the uterine cavity,which ensures ultrasound images(cheap medical instrument).When biopsy is demanded,this catheter will adsorb endometrium tissue for diagnosis.Using this kind of catheter,two tasks of ultrasonic inspection and biopsy can be simplified as one. Doctors who have used GoldsteinSonoBiopsy confirm that ultrasound diagnosis(bovine ultrasound machine) and biopsy done with the help of GoldsteinSonoBiopsy only cost 70% of the regular time and the discomfort of patients may also alleviated. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Cook-Medical-developed-innovative-metrorrhagia-examining-catheter.html
It's claimed that the uniquely shaped sealing component of GoldsteinSonoBiopsy can prevent the overflow of saline solution that flows into the uterine cavity,which ensures ultrasound images(cheap medical instrument).When biopsy is demanded,this catheter will adsorb endometrium tissue for diagnosis.Using this kind of catheter,two tasks of ultrasonic inspection and biopsy can be simplified as one. Doctors who have used GoldsteinSonoBiopsy confirm that ultrasound diagnosis(bovine ultrasound machine) and biopsy done with the help of GoldsteinSonoBiopsy only cost 70% of the regular time and the discomfort of patients may also alleviated. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Cook-Medical-developed-innovative-metrorrhagia-examining-catheter.html
The kind of catheter MSL introduced on Aug.6th.The innovative feature is that doctors can acquire clearer ultrasonic images(bovine ultrasound machines) when inspecting metrorrhagia patients by injecting saline solution into uterine cavity.During the process,doctors can also sample to do endometrium biopsy as the actual inspection condition may require. Metrorrhagia is one of the most common diseases that lead females to paediatrics and obstetrics.In America,there are up to 10,000,000 patients turn to doctors for metrorrhagia per year.It can be one symptom of some serious illness,such as endometrial carcinoma,leukemia.And even a common metrorrhagia patient can suffer anemia if not timely treated,not to mention that at the same time,daily life of the patient can be affected badly.  It's claimed that the uniquely shaped sealing component of GoldsteinSonoBiopsy can prevent the overflow of saline solution that flows into the uterine cavity,which ensures ultrasound images(cheap medical instrument).When biopsy is demanded,this catheter will adsorb endometrium tissue for diagnosis.Using this kind of catheter,two tasks of ultrasonic inspection and biopsy can be simplified as one. Doctors who have used GoldsteinSonoBiopsy confirm that ultrasound diagnosis(bovine ultrasound machine) and biopsy done with the help of GoldsteinSonoBiopsy only cost 70% of the regular time and the discomfort of patients may also alleviated. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Cook-Medical-developed-innovative-metrorrhagia-examining-catheter.html
It's claimed that the uniquely shaped sealing component of GoldsteinSonoBiopsy can prevent the overflow of saline solution that flows into the uterine cavity,which ensures ultrasound images(cheap medical instrument).When biopsy is demanded,this catheter will adsorb endometrium tissue for diagnosis.Using this kind of catheter,two tasks of ultrasonic inspection and biopsy can be simplified as one. Doctors who have used GoldsteinSonoBiopsy confirm that ultrasound diagnosis(bovine ultrasound machine) and biopsy done with the help of GoldsteinSonoBiopsy only cost 70% of the regular time and the discomfort of patients may also alleviated. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/Cook-Medical-developed-innovative-metrorrhagia-examining-catheter.html
6 août,la société a annoncé un nom de cathéter est GoldsteinSonoBiopsy,sa capacité d'innovation s'est traduite par ce que lorsque l'opérateur est utiliser ce produit pour examiner lés patients,il peut faire la solution saline injectée par le cathéter à la cavité utérine ,et puis obtenir meilleures images échographiques(buy cheap ultrasound equipment),dans ce processus,le médecin peut faire échantillonnage de biopsie de l'endomètre selon l'inspection.  La hémorragie utérine est la plus commune d'une espèce de maladies gynécologie et obstétrique,lès patients en raison de hémorragie utérine a déjà franchi 10 millions de personnes par année aux Etats-Unis La hémorragie utérine peut être un symptôme de certaines maladies graves,telles que le cancer de l'endomètre,de la leucémie,même les patients avec hémorragie utérine normale,traitement pas opportun peut causer de l'anémie(color doppler usb ultrasound box),la vie quotidienne des patients est grandement affectée. Selon les explication,composants étanches a forme spéciale de ce cathéter ,elle peut éviter le débordement de solution saline qui entre dans la cavité utérine,assurer d'excellentes images de l'échographie,lorsqu'une biopsie est nécessaire,ce cathéter peut absorber adsorbé tissu endométrial,pour faire un diagnostic(price of ultrasound machine) .Avec ce cathéter ,l'échographie et biopsie deux grandes tâches en un seul. Selon le médecin américain qui a utilisé ce cathéter introduit,par rapport à d'autres technologies de cathéter,le temps de diagnostic à ultrasons et une biopsie par cathéter GoldsteinSonoBiopsy pourrait être de 30% inférieure,et l'inconfort du patient réduit considérablement. The article comes from: http://www.medicalequipment-msl.com/htm/medical-device-book/La-soci%C3%A9t%C3%A9-a-d%C3%A9velopp%C3%A9-le-cath%C3%A9ter-pour-l'examen-de-h%C3%A9morragie-ut%C3%A9rine-avec-l'innovation-et-la-technologie.html
La hémorragie utérine est la plus commune d'une espèce de maladies gynécologie et obstétrique,lès patients en raison de hémorragie utérine a déjà franchi 10 millions de personnes par année aux Etats-Unis La hémorragie utérine peut être un symptôme de certaines maladies graves,telles que le cancer de l'endomètre,de la leucémie,même les patients avec hémorragie utérine normale,traitement pas opportun peut causer de l'anémie(color doppler usb ultrasound box),la vie quotidienne des patients est grandement affectée. Selon les explication,composants étanches a forme spéciale de ce cathéter ,elle peut éviter le débordement de solution saline qui entre dans la cavité utérine,assurer d'excellentes images de l'échographie,lorsqu'une biopsie est nécessaire,ce cathéter peut absorber adsorbé tissu endométrial,pour faire un diagnostic(price of ultrasound machine) .Avec ce cathéter ,l'échographie et biopsie deux grandes tâches en un seul. Selon le médecin américain qui a utilisé ce cathéter introduit,par rapport à d'autres technologies de cathéter,le temps de diagnostic à ultrasons et une biopsie par cathéter GoldsteinSonoBiopsy pourrait être de 30% inférieure,et l'inconfort du patient réduit considérablement. The article comes from: http://www.medicalequipment-msl.com/htm/medical-device-book/La-soci%C3%A9t%C3%A9-a-d%C3%A9velopp%C3%A9-le-cath%C3%A9ter-pour-l'examen-de-h%C3%A9morragie-ut%C3%A9rine-avec-l'innovation-et-la-technologie.html
6 août,la société a annoncé un nom de cathéter est GoldsteinSonoBiopsy,sa capacité d'innovation s'est traduite par ce que lorsque l'opérateur est utiliser ce produit pour examiner lés patients,il peut faire la solution saline injectée par le cathéter à la cavité utérine ,et puis obtenir meilleures images échographiques(buy cheap ultrasound equipment),dans ce processus,le médecin peut faire échantillonnage de biopsie de l'endomètre selon l'inspection.  La hémorragie utérine est la plus commune d'une espèce de maladies gynécologie et obstétrique,lès patients en raison de hémorragie utérine a déjà franchi 10 millions de personnes par année aux Etats-Unis La hémorragie utérine peut être un symptôme de certaines maladies graves,telles que le cancer de l'endomètre,de la leucémie,même les patients avec hémorragie utérine normale,traitement pas opportun peut causer de l'anémie(color doppler usb ultrasound box),la vie quotidienne des patients est grandement affectée. Selon les explication,composants étanches a forme spéciale de ce cathéter ,elle peut éviter le débordement de solution saline qui entre dans la cavité utérine,assurer d'excellentes images de l'échographie,lorsqu'une biopsie est nécessaire,ce cathéter peut absorber adsorbé tissu endométrial,pour faire un diagnostic(price of ultrasound machine) .Avec ce cathéter ,l'échographie et biopsie deux grandes tâches en un seul. Selon le médecin américain qui a utilisé ce cathéter introduit,par rapport à d'autres technologies de cathéter,le temps de diagnostic à ultrasons et une biopsie par cathéter GoldsteinSonoBiopsy pourrait être de 30% inférieure,et l'inconfort du patient réduit considérablement. The article comes from: http://www.medicalequipment-msl.com/htm/medical-device-book/La-soci%C3%A9t%C3%A9-a-d%C3%A9velopp%C3%A9-le-cath%C3%A9ter-pour-l'examen-de-h%C3%A9morragie-ut%C3%A9rine-avec-l'innovation-et-la-technologie.html
La hémorragie utérine est la plus commune d'une espèce de maladies gynécologie et obstétrique,lès patients en raison de hémorragie utérine a déjà franchi 10 millions de personnes par année aux Etats-Unis La hémorragie utérine peut être un symptôme de certaines maladies graves,telles que le cancer de l'endomètre,de la leucémie,même les patients avec hémorragie utérine normale,traitement pas opportun peut causer de l'anémie(color doppler usb ultrasound box),la vie quotidienne des patients est grandement affectée. Selon les explication,composants étanches a forme spéciale de ce cathéter ,elle peut éviter le débordement de solution saline qui entre dans la cavité utérine,assurer d'excellentes images de l'échographie,lorsqu'une biopsie est nécessaire,ce cathéter peut absorber adsorbé tissu endométrial,pour faire un diagnostic(price of ultrasound machine) .Avec ce cathéter ,l'échographie et biopsie deux grandes tâches en un seul. Selon le médecin américain qui a utilisé ce cathéter introduit,par rapport à d'autres technologies de cathéter,le temps de diagnostic à ultrasons et une biopsie par cathéter GoldsteinSonoBiopsy pourrait être de 30% inférieure,et l'inconfort du patient réduit considérablement. The article comes from: http://www.medicalequipment-msl.com/htm/medical-device-book/La-soci%C3%A9t%C3%A9-a-d%C3%A9velopp%C3%A9-le-cath%C3%A9ter-pour-l'examen-de-h%C3%A9morragie-ut%C3%A9rine-avec-l'innovation-et-la-technologie.html
There have a new progress of Chinese hyperthermia technology(look for cheap xray machine mobile prices).At present,Beijing Rende Sheng Technology Company signed a contract with Hong Kong Taihe company,their going to cooperate to put the RDS HIFU tumor therapy machine into application platform from R & D platform. 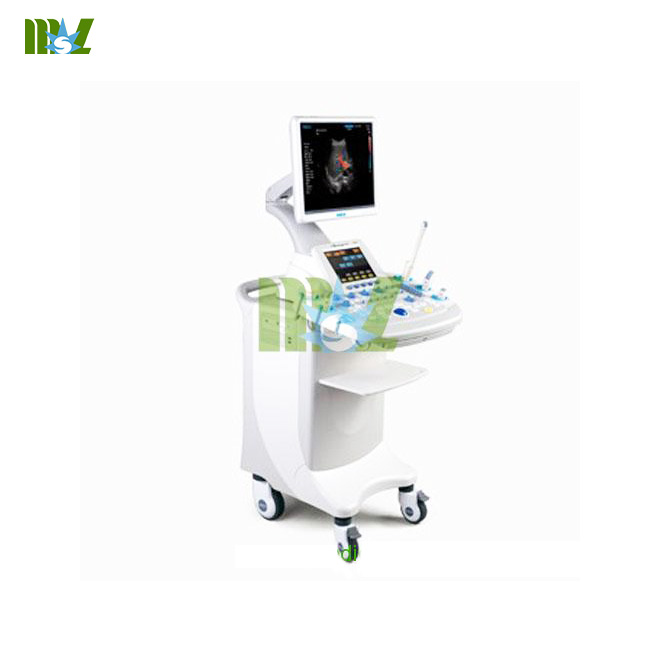 The main researcher Renai Qing who is the major developers of (ultrasound machines for sale)High Intensity Focused Ultrasound Knife said,this technology is a high-tech products with independent intellectual property rights in China.RDS high intensity focused ultrasound tumor therapy machine,using ultrasonic energy,give full play to the ultrasound fat but not hot,Penetrating well,pointing to strong,focus can be good.Under the precise computer control,through the transducer,it can focus the beam from different directions to the same tumor site and converted into heat,about 0.25 seconds in the tumor treatment the temperature will reaches 70 ℃~100 ℃,making the tumor cell degeneration ,necrosis,for therapeutic purposes.Little use in the treatment of a line,the line wire into the surface,all things into treatment accumulate body kill tumor cells sequentially(blood analyzer for sale),and is accurate to the killing of focus 6 cells.Since the treatment without anesthesia,the patient is awake,it increased safety of the treatment. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/The-new-type-high-Intensity-Focused-Ultrasound-Knife-of-China-is-going-to-be-applied-to-clinical.html
The main researcher Renai Qing who is the major developers of (ultrasound machines for sale)High Intensity Focused Ultrasound Knife said,this technology is a high-tech products with independent intellectual property rights in China.RDS high intensity focused ultrasound tumor therapy machine,using ultrasonic energy,give full play to the ultrasound fat but not hot,Penetrating well,pointing to strong,focus can be good.Under the precise computer control,through the transducer,it can focus the beam from different directions to the same tumor site and converted into heat,about 0.25 seconds in the tumor treatment the temperature will reaches 70 ℃~100 ℃,making the tumor cell degeneration ,necrosis,for therapeutic purposes.Little use in the treatment of a line,the line wire into the surface,all things into treatment accumulate body kill tumor cells sequentially(blood analyzer for sale),and is accurate to the killing of focus 6 cells.Since the treatment without anesthesia,the patient is awake,it increased safety of the treatment. The article comes from:http://www.medicalequipment-msl.com/htm/medical-device-book/The-new-type-high-Intensity-Focused-Ultrasound-Knife-of-China-is-going-to-be-applied-to-clinical.html








